Description
Drug Info: ApoThera Drug Disease Clinical Support App
Storage
– Refrigerate at 2-8ºC (36-46ºF)
– If needed, each single-dose pen can be stored unrefrigerated at temperatures not to exceed 30ºC (86ºF) for up to 21 days
– Do not freeze
– Protect from light
– If needed, each single-dose pen can be stored unrefrigerated at temperatures not to exceed 30ºC (86ºF) for up to 21 days
– Do not freeze
– Protect from light
Missed Dose
<4 Days (96 hr)
– Administer as soon as possible after missed dose
– Administer as soon as possible after missed dose
>4 days
– Skip missed dose and administer next dose on regularly scheduled day
– In each case, patients can resume their regular once weekly dosing schedule
– Skip missed dose and administer next dose on regularly scheduled day
– In each case, patients can resume their regular once weekly dosing schedule
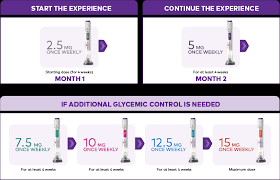
How Mounjaro Works

SC Administration
– Administer SC in abdomen, thigh, or upper arm
– Rotate injection site with each dose
– Administer once weekly, at any time of day, with or without meals
– Change day of weekly administration: May be changed, if necessary, as long as time between the 2 doses is at least 3 days (72 hr)
– Rotate injection site with each dose
– Administer once weekly, at any time of day, with or without meals
– Change day of weekly administration: May be changed, if necessary, as long as time between the 2 doses is at least 3 days (72 hr)
Instructions for Mounjaro SC Injection (Video, Click Play)





SC Preparation
– Inspect visually before use
– Solution should appear clear and colorless to slightly yellow; discard if particulate matter or discoloration observed
– Solution should appear clear and colorless to slightly yellow; discard if particulate matter or discoloration observed
The Most Common Side Effects
– Nausea
– Diarrhea
– Decreased appetite
– Vomiting
– Constipation
– Indigestion
– Stomach (abdominal) pain
– The most common adverse reactions reported in ≥5% of Mounjaro-treated patients in placebo-controlled trials were nausea, diarrhea, decreased appetite, vomiting, constipation, dyspepsia, and abdominal pain.
Kidney Problems (Kidney Failure)
– In people who have kidney problems, diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration) which may cause kidney problems to get worse
– It is important for you to drink fluids to help reduce your chance of dehydration
– Mounjaro has been associated with gastrointestinal adverse reactions, which include nausea, vomiting, and diarrhea. These events may lead to dehydration, which if severe could cause acute kidney injury. In patients treated with GLP-1 receptor agonists, there have been post-marketing reports of acute kidney injury and worsening of chronic renal failure, sometimes requiring hemodialysis. Some of these events have been reported in patients without known underlying renal disease. A majority of reported events occurred in patients who had experienced nausea, vomiting, diarrhea, or dehydration. Monitor renal function when initiating or escalating doses of Mounjaro in patients with renal impairment reporting severe adverse gastrointestinal reactions.
Severe Stomach Problems
– Stomach problems, sometimes severe, have been reported in people who use MOUNJARO
– Tell your healthcare provider if you have stomach problems that are severe or will not go away
Changes in Vision
– Tell your healthcare provider if you have changes in vision during treatment with MOUNJARO.
– Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy.
– Mounjaro has not been studied in patients with non-proliferative diabetic retinopathy requiring acute therapy, proliferative diabetic retinopathy, or diabetic macular edema
– Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy.
Gallbladder Problems
– Gallbladder problems have happened in some people who use MOUNJARO
– In clinical trials, acute gallbladder disease was reported by 0.6% of Mounjaro-treated patients and 0% of placebo-treated patients. If cholelithiasis is suspected, gallbladder diagnostic studies and appropriate clinical follow-up are indicated
– Tell your healthcare provider right away if you get symptoms of gallbladder problems which may include:
- pain in your upper stomach (abdomen)
- fever
- yellowing of skin or eyes (jaundice)
- clay-colored stools
Low Blood Sugar (Hypoglycemia)
– Your risk for getting low blood sugar may be higher if you use MOUNJARO with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin
– Signs and symptoms of low blood sugar may include:
- dizziness or light-headedness
- sweating
- confusion or drowsiness
- headache
- blurred vision
- slurred speech
- shakiness
- fast heartbeat
- anxiety, irritability, or mood changes
- hunger
- weakness
- feeling jittery
– Concomitant use with an insulin secretagogue (e.g., sulfonylurea) or insulin may increase the risk of hypoglycemia, including severe hypoglycemia
– The risk of hypoglycemia may be lowered by reducing the dose of sulfonylurea (or other concomitantly administered insulin secretagogue) or insulin.
– Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.
Inflammation of Pancreas (Pancreatitis)
– Stop using MOUNJARO and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your abdomen to your back.
Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists. Pancreatitis has been reported in Mounjaro clinical trials. Mounjaro has not been studied in patients with a prior history of pancreatitis. It is unknown if patients with a history of pancreatitis are at higher risk for development of pancreatitis on Mounjaro. Observe patients for signs and symptoms, including persistent severe abdominal pain sometimes radiating to the back, which may or may not be accompanied by vomiting. If pancreatitis is suspected, discontinue Mounjaro and initiate appropriate management.
Pregnancy
– Tell your healthcare provider if you are pregnant or plan to become pregnant
– Tell your healthcare provider if you become pregnant while using MOUNJARO
– Limited data on MOUNJARO use in pregnant women are available to inform on drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes
– Based on animal reproduction studies, there may be risks to the fetus from exposure to MOUNJARO
– Use only if potential benefit justifies the potential risk to the fetus
Lactation
– It is not known if MOUNJARO passes into your breast milk
– Talk to your healthcare provider about the best way to feed your baby while using MOUNJARO
– There are no data on the presence of MOUNJARO in human milk, the effects on the breastfed infant, or the effects on milk production.
– The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for MOUNJARO and any potential adverse effects on the breastfed infant from MOUNJARO or from the underlying maternal condition
Females of Reproductive Potential
– Birth control pills by mouth may not work as well while using MOUNJARO
– If you take birth control pills by mouth, your healthcare provider may recommend another type of birth control for 4 weeks after you start MOUNJARO and for 4 weeks after each increase in your dose of MOUNJARO
– Talk to your healthcare provider about birth control methods that may be right for you while using MOUNJARO
– Advise females using oral hormonal contraceptives to switch to a non-oral contraceptive method, or add a barrier method of contraception for 4 weeks after initiation and for 4 weeks after each dose escalation.
Active Ingredient
– Tirzepatide (Mounjaro)
Inactive Ingredients
Inactive Ingredients
– Sodium chloride
– Sodium phosphate dibasic heptahydrate
– Water for injection
– Hydrochloric acid solution and/or sodium hydroxide solution may have been added to adjust the pH
Contraindications
– A personal or family history of medullary thyroid carcinoma (MTC) or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2)
– Known serious hypersensitivity to tirzepatide or any of the excipients in MOUNJARO. Serious hypersensitivity reactions, including anaphylaxis and angioedema, have been reported with MOUNJARO
Related
Tags: Cardiovascular Disease Prevention - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Atherton | Mounjaro (tirzepatide) Injection Atherton | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Atherton | Mounjaro PreFilled Pens Injection Atherton | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Avila Beach - Mounjaro 2.5mg In Stock Avila Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Avila Beach | Mounjaro (tirzepatide) Injection Avila Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Avila Beach | Mounjaro PreFilled Pens Injection Avila Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Balboa Island - Mounjaro 2.5mg In Stock Balboa Island | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Balboa Island | Mounjaro (tirzepatide) Injection Balboa Island | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Balboa Island | Mounjaro PreFilled Pens Injection Balboa Island | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Balboa Peninsula | Mounjaro PreFilled Pens Injection Balboa Peninsula | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Bel Air - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Bel Air | Mounjaro (tirzepatide) Injection Bel Air | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Bel Air | Mounjaro PreFilled Pens Injection Bel Air | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Belmont | Mounjaro (tirzepatide) Injection Belmont | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Belmont | Mounjaro 2.5mg In Stock Belmont | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Belmont | Mounjaro PreFilled Pens Injection Belmont | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Belvedere | Mounjaro PreFilled Pens Injection Belvedere | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Belvedere | Mounjaro PreFilled Pens Injection Injection Belvedere | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Beverly Glen - Mounjaro 2.5mg In Stock Beverly Glen | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Beverly Glen | Mounjaro (tirzepatide) Injection Beverly Glen | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Beverly Glen | Mounjaro PreFilled Pens Injection Beverly Glen | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Beverly Hills - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Beverly Hills | Mounjaro (tirzepatide) Injection Beverly Hills | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Beverly Hills | Mounjaro PreFilled Pens Injection Beverly Hills | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Big Sur | Mounjaro PreFilled Pens Injection Big Sur | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Brentwood - Mounjaro 2.5mg In Stock Brentwood | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Brentwood | Mounjaro (tirzepatide) Injection Brentwood | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Brentwood | Mounjaro PreFilled Pens Injection Brentwood | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Bridgeport | Mounjaro (tirzepatide) Injection Bridgeport | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Bridgeport | Mounjaro PreFilled Pens Injection Bridgeport | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Burlingame | Mounjaro PreFilled Pens Injection Burlingame | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Calabasas | Mounjaro (tirzepatide) Injection Calabasas | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Calabasas | Mounjaro PreFilled Pens Injection Calabasas | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Capistrano Beach | Mounjaro (tirzepatide) Injection Capistrano Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Capistrano Beach | Mounjaro 2.5mg In Stock Capistrano Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Capistrano Beach | Mounjaro PreFilled Pens Injection Capistrano Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Carlsbad | Mounjaro PreFilled Pens Injection Carlsbad | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Carpinteria | Mounjaro (tirzepatide) Injection Carpinteria | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Carpinteria | Mounjaro 2.5mg In Stock Carpinteria | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Carpinteria | Mounjaro PreFilled Pens Injection Carpinteria | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Chappaqua | Mounjaro (tirzepatide) Injection Chappaqua | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Chappaqua | Mounjaro PreFilled Pens Injection Chappaqua | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Concord | Mounjaro PreFilled Pens Injection Concord | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Corona Del Mar | Mounjaro (tirzepatide) Injection Corona Del Mar | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Corona Del Mar | Mounjaro PreFilled Pens Injection Corona Del Mar | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Coronado | Mounjaro (tirzepatide) Injection Coronado | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Coronado | Mounjaro 2.5mg In Stock Coronado | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Coronado | Mounjaro PreFilled Pens Injection Coronado | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Corte Madera | Mounjaro PreFilled Pens Injection Corte Madera | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Crystal Cove - Mounjaro 2.5mg In Stock Crystal Cove | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Crystal Cove | Mounjaro (tirzepatide) Injection Crystal Cove | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Crystal Cove | Mounjaro PreFilled Pens Injection Crystal Cove | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Cupertino | Mounjaro (tirzepatide) Injection Cupertino | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Cupertino | Mounjaro PreFilled Pens Injection Cupertino | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Dana Point | Mounjaro PreFilled Pens Injection Dana Point | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Del Mar Heights | Mounjaro (tirzepatide) Injection Del Mar Heights | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Del Mar Heights | Mounjaro PreFilled Pens Injection Del Mar Heights | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine East Hampton | Mounjaro PreFilled Pens Injection East Hampton | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Emerald Bay | Mounjaro PreFilled Pens Injection Emerald Bay | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Encinitas | Mounjaro PreFilled Pens Injection Encinitas | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Fairbanks Ranch | Mounjaro PreFilled Pens Injection Fairbanks Ranch | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Foster City | Mounjaro (tirzepatide) Injection Foster City | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Foster City | Mounjaro 2.5mg In Stock Foster City | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Foster City | Mounjaro PreFilled Pens Injection Foster City | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Garden City | Mounjaro (tirzepatide) Injection Garden City | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Garden City | Mounjaro PreFilled Pens Injection Garden City | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Goleta | Mounjaro (tirzepatide) Injection Goleta | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Greenwich | Mounjaro (tirzepatide) Injection Greenwich | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Greenwich | Mounjaro PreFilled Pens Injection Greenwich | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Greenwich Village | Mounjaro PreFilled Pens Injection Greenwich Village | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Half Moon Bay | Mounjaro PreFilled Pens Injection Half Moon Bay | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Hampton Bays | Mounjaro (tirzepatide) Injection Hampton Bays | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Hampton Bays | Mounjaro 2.5mg In Stock Hampton Bays | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Hampton Bays | Mounjaro PreFilled Pens Injection Hampton Bays | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Hidden Hills | Mounjaro (tirzepatide) Injection Hidden Hills | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Hidden Hills | Mounjaro PreFilled Pens Injection Hidden Hills | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Hidden Valley | Mounjaro (tirzepatide) Injection Hidden Valley | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Hidden Valley | Mounjaro PreFilled Pens Injection Hidden Valley | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Hillsborough | Mounjaro PreFilled Pens Injection Hillsborough | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Hollywood | Mounjaro PreFilled Pens Injection Hollywood | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Hollywood Hills | Mounjaro PreFilled Pens Injection Hollywood Hills | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Hope Ranch | Mounjaro (tirzepatide) Injection Hope Ranch | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Hope Ranch | Mounjaro 2.5mg In Stock Hope Ranch | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Hope Ranch | Mounjaro PreFilled Pens Injection Goleta | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Hope Ranch | Mounjaro PreFilled Pens Injection Hope Ranch | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine La Jolla | Mounjaro (tirzepatide) Injection La Jolla | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine La Jolla | Mounjaro 2.5mg In Stock La Jolla | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine La Jolla | Mounjaro PreFilled Pens Injection La Jolla | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine La Jolla Shores - Mounjaro 2.5mg In Stock La Jolla Shores | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine La Jolla Shores | Mounjaro (tirzepatide) Injection La Jolla Shores | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine La Jolla Shores | Mounjaro PreFilled Pens Injection La Jolla Shores | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Laguna Beach - Mounjaro 2.5mg In Stock Laguna Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Laguna Beach | Mounjaro (tirzepatide) Injection Laguna Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Laguna Beach | Mounjaro PreFilled Pens Injection Laguna Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Larkspur | Mounjaro (tirzepatide) Injection Larkspur | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Larkspur | Mounjaro PreFilled Pens Injection Larkspur | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Leucadia | Mounjaro PreFilled Pens Injection Leucadia | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Long Island - Mounjaro 2.5mg In Stock Long Island | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Long Island | Mounjaro (tirzepatide) Injection Long Island | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Long Island | Mounjaro PreFilled Pens Injection Long Island | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Los Gatos | Mounjaro PreFilled Pens Injection Los Gatos | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Lower Manhattan | Mounjaro PreFilled Pens Injection Lower Manhattan | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Malibu - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Malibu - Mounjaro 2.5mg In Stock Malibu | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Malibu | Mounjaro (tirzepatide) Injection Malibu | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Malibu | Mounjaro PreFilled Pens Injection Malibu | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Manhattan - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Manhattan | Mounjaro (tirzepatide) Injection Manhattan | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Manhattan | Mounjaro PreFilled Pens Injection Manhattan | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Manhattan Beach | Mounjaro PreFilled Pens Injection Manhattan Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Manhattan Financial District - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Manhattan Financial District | Mounjaro (tirzepatide) Injection Manhattan Financial District | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Manhattan Financial District | Mounjaro PreFilled Pens Injection Manhattan Financial District | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Manzanita | Mounjaro PreFilled Pens Injection Manzanita | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Marin County - Mounjaro 2.5mg In Stock Marin County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Marin County | Mounjaro (tirzepatide) Injection Marin County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Marin County | Mounjaro PreFilled Pens Injection Marin County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Massapequa - Mounjaro 2.5mg In Stock Massapequa | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Massapequa | Mounjaro (tirzepatide) Injection Massapequa | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Massapequa | Mounjaro PreFilled Pens Injection Massapequa | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Melno Park | Mounjaro PreFilled Pens Injection Melno Park | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Mill Valley - Mounjaro 2.5mg In Stock Mill Valley | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Mill Valley | Mounjaro (tirzepatide) Injection Mill Valley | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Mill Valley | Mounjaro PreFilled Pens Injection Mill Valley | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Millbrae | Mounjaro PreFilled Pens Injection Millbrae | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Mills Estates | Mounjaro PreFilled Pens Injection Mill Estates | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Mira Mesa | Mounjaro PreFilled Pens Injection Mira Mesa | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Monarch Beach - Mounjaro 2.5mg In Stock Monarch Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Monarch Beach | Mounjaro (tirzepatide) Injection Monarch Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Monarch Beach | Mounjaro PreFilled Pens Injection Monarch Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Montauk | Mounjaro PreFilled Pens Injection Montauk | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Montecito - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Montecito - Mounjaro 2.5mg In Stock Montecito | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Montecito | Mounjaro (tirzepatide) Injection Montecito | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Montecito | Mounjaro PreFilled Pens Injection Montecito | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Monterey | Mounjaro PreFilled Pens Injection Monterey | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Moss Beach | Mounjaro PreFilled Pens Injection Moss Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Mountain View | Mounjaro (tirzepatide) Injection Mountain View | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Mountain View | Mounjaro 2.5mg In Stock Mountain View | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Mountain View | Mounjaro PreFilled Pens Injection Mountain View | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Napa | Mounjaro PreFilled Pens Injection Napa | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Nassau County | Mounjaro (tirzepatide) Injection Nassau County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Nassau County | Mounjaro PreFilled Pens Injection Nassau County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Newport Beach - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Newport Beach | Mounjaro (tirzepatide) Injection Newport Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Newport Beach | Mounjaro PreFilled Pens Injection Newport Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Newport Coast - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Newport Coast | Mounjaro (tirzepatide) Injection Newport Coast | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Newport Coast | Mounjaro PreFilled Pens Injection Newport Coast | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Oceanside | Mounjaro PreFilled Pens Injection Oceanside | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Ojai | Mounjaro (tirzepatide) Injection Ojai | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Ojai | Mounjaro 2.5mg In Stock Injection Ojai | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Ojai | Mounjaro PreFilled Pens Injection Ojai | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Oyster Bay | Mounjaro PreFilled Pens Injection Oyster Bay | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Pacific Beach | Mounjaro (tirzepatide) Injection Pacific Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Pacific Beach | Mounjaro 2.5mg In Stock Pacific Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Pacific Beach | Mounjaro PreFilled Pens Injection Pacific Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Pacific Palisades - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Pacific Palisades | Mounjaro (tirzepatide) Injection Pacific Palisades | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Pacific Palisades | Mounjaro PreFilled Pens Injection Pacific Palisades | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Pacifica | Mounjaro (tirzepatide) Injection Pacifica | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Pacifica | Mounjaro 2.5mg In Stock Pacifica | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Pacifica | Mounjaro PreFilled Pens Injection Pacifica | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Palm Desert | Mounjaro (tirzepatide) Injection Palm Desert | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Palm Desert | Mounjaro 2.5mg In Stock Palm Desert | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Palm Desert | Mounjaro PreFilled Pens Injection Palm Desert | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Palm Springs | Mounjaro (tirzepatide) Injection Palm Springs | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Palm Springs | Mounjaro 2.5mg In Stock Palm Springs | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Palm Springs | Mounjaro PreFilled Pens Injection Palm Springs | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Palo Alto | Mounjaro PreFilled Pens Injection Palo Alto | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Palos Verdes Peninsula | Mounjaro PreFilled Pens Injection Palos Verdes Peninsula | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Paramount Ranch | Mounjaro PreFilled Pens Injection Paramount Ranch | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Patchogue - Mounjaro 2.5mg In Stock Patchogue | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Patchogue | Mounjaro (tirzepatide) Injection Patchogue | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Patchogue | Mounjaro PreFilled Pens Injection Patchogue | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Pebble Beach | Mounjaro PreFilled Pens Injection Pebble Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Pismo Beach | Mounjaro PreFilled Pens Injection Pismo Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Point Loma | Mounjaro (tirzepatide) Injection Point Loma | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Point Loma | Mounjaro 2.5mg In Stock Point Loma | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Point Loma | Mounjaro PreFilled Pens Injection Point Loma | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Port Hueneme | Mounjaro (tirzepatide) Injection Port Hueneme | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Port Hueneme | Mounjaro 2.5mg In Stock Port Hueneme | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Port Hueneme | Mounjaro PreFilled Pens Injection Port Hueneme | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Rancho Bernardo - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Rancho Bernardo | Mounjaro (tirzepatide) Injection Rancho Bernardo | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Rancho Bernardo | Mounjaro PreFilled Pens Injection Rancho Bernardo | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Rancho Encantada | Mounjaro PreFilled Pens Injection Rancho Encantada | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Rancho Palos Verdes | Mounjaro (tirzepatide) Injection Rancho Palos Verdes | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Rancho Palos Verdes | Mounjaro PreFilled Pens Injection Rancho Palos Verdes | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Rancho Peñasquitos - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Rancho Peñasquitos | Mounjaro (tirzepatide) Injection Rancho Penasquitos | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Rancho Peñasquitos | Mounjaro PreFilled Pens Injection Rancho Penasquitos | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Rancho Santa Fe - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Rancho Santa Fe | Mounjaro (tirzepatide) Injection Rancho Santa Fe | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Rancho Santa Fe | Mounjaro PreFilled Pens Injection Rancho Santa Fe | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Redwood City | Mounjaro (tirzepatide) Injection Redwood City | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Redwood City | Mounjaro 2.5mg In Stock Redwood City | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Redwood City | Mounjaro PreFilled Pens Injection Redwood City | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Redwood Shores | Mounjaro PreFilled Pens Injection Redwood Shores | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Rincon Beach | Mounjaro PreFilled Pens Injection Rincon Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Rolling Hills | Mounjaro PreFilled Pens Injection Rolling Hills | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine San Bruno | Mounjaro PreFilled Pens Injection San Bruno | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine San Carlos | Mounjaro PreFilled Pens Injection San Carlos | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine San Clemente | Mounjaro (tirzepatide) Injection San Clemente | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine San Clemente | Mounjaro 2.5mg In Stock San Clemente | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine San Clemente | Mounjaro PreFilled Pens Injection San Clemente | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine San Mateo County | Mounjaro (tirzepatide) Injection San Mateo County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine San Mateo County | Mounjaro PreFilled Pens Injection San Mateo County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine San Rafael | Mounjaro PreFilled Pens Injection San Rafael | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Santa Barbara - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Santa Barbara | Mounjaro (tirzepatide) Injection Santa Barbara | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Santa Barbara | Mounjaro PreFilled Pens Injection Santa Barbara | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Santa Cruz | Mounjaro PreFilled Pens Injection Santa Cruz | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Santa Monica | Mounjaro PreFilled Pens Injection Santa Monica | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Saratoga | Mounjaro PreFilled Pens Injection Saratoga | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Sausalito - Mounjaro 2.5mg In Stock Sausalito | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Sausalito | Mounjaro (tirzepatide) Injection Sausalito | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Sausalito | Mounjaro PreFilled Pens Injection Sausalito | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Scripps Ranch | Mounjaro PreFilled Pens Injection Scripps Ranch | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Shell Beach | Mounjaro PreFilled Pens Injection Shell Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Shelter Island | Mounjaro PreFilled Pens Injection Shelter Island | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Silicon Valley - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Silicon Valley | Mounjaro (tirzepatide) Injection Silicon Valley | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Silicon Valley | Mounjaro PreFilled Pens Injection Silicon Valley | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Solana Beach | Mounjaro PreFilled Pens Injection Solana Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Sonoma | Mounjaro PreFilled Pens Injection Sonoma | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Sorrento Valley | Mounjaro PreFilled Pens Injection Sorrento Valley | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine South Pasadena | Mounjaro PreFilled Pens Injection South Pasadena | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine South San Francisco | Mounjaro (tirzepatide) Injection South San Francisco | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine South San Francisco | Mounjaro PreFilled Pens Injection South San Francisco | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Southampton | Mounjaro PreFilled Pens Injection Southampton | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Stinson Beach | Mounjaro PreFilled Pens Injection Stinson Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Studio City | Mounjaro (tirzepatide) Injection Studio City | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Studio City | Mounjaro 2.5mg In Stock Studio City | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Studio City | Mounjaro PreFilled Pens Injection Studio City | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Suffolk County | Mounjaro (tirzepatide) Injection Suffolk County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Suffolk County | Mounjaro PreFilled Pens Injection Suffolk County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Summerland | Mounjaro (tirzepatide) Injection Summerland | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Summerland | Mounjaro PreFilled Pens Injection Summerland | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Sunnyvale | Mounjaro PreFilled Pens Injection Sunnyvale | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Tiburon | Mounjaro PreFilled Pens Injection Tiburon | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Torrey Pines | Mounjaro (tirzepatide) Injection Torrey Pines | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Torrey Pines | Mounjaro 2.5mg In Stock Torrey Pines | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Torrey Pines | Mounjaro PreFilled Pens Injection Torrey Pines | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Tribeca | Mounjaro PreFilled Pens Injection Tribeca | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Tuckahoe | Mounjaro PreFilled Pens Injection Tuckahoe | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Venice Beach - Mounjaro 2.5mg In Stock Venice Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Venice Beach | Mounjaro (tirzepatide) Injection Venice Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Venice Beach | Mounjaro PreFilled Pens Injection Venice Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Ventura Beach | Mounjaro PreFilled Pens Injection Ventura Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Village of La Jolla | Mounjaro PreFilled Pens Injection Village of La Jolla | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Wall Street | Mounjaro (tirzepatide) Injection Wall Street | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Wall Street | Mounjaro PreFilled Pens Injection Wall Street | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Westhampton Beach | Mounjaro PreFilled Pens Injection Westhampton Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Westport | Mounjaro (tirzepatide) Injection Westport | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Westport | Mounjaro PreFilled Pens Injection Westport | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Concierge Medicine Westwood | Mounjaro PreFilled Pens Injection Westwood | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Diabetes - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Diabetes Mellitus - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Functional Medicine - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, How to Get Mounjaro® (tirzepatide) - A Lilly Medicine - Concierge Medicine Cupertino - Mounjaro 2.5mg In Stock Cupertino | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, How to Get Mounjaro® (tirzepatide) - A Lilly Medicine - Concierge Medicine Wall Street - Mounjaro 2.5mg In Stock Wall Street | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Integrative Medicine - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Atherton | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Avila Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Balboa Island | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Bel Air | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Beverly Glen | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg in Stock Beverly Hills | ApoThera - Concierge Specialty Pharmacy, Mounjaro 2.5mg In Stock Brentwood | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Corona Del Mar | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Crystal Cove | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Dana Point | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Del Mar Heights | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock East Hampton | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Emerald Bay | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Goleta | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Hampton Bays | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Hamptons | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock La Jolla | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock La Jolla Shores | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Laguna Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Long Island | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Los Angeles | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Malibu | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Manhattan | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Manhattan Financial District | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Marin County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Massapequa | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Mill Valley | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Monarch Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Montecito | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock New York City | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Newport Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Newport Coast | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Pacific Palisades | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Palos Verdes Peninsula | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Patchogue | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Rancho Bernardo | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Rancho Palos Verdes | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg in stock Rancho Penasquitos | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Rancho Santa Fe | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock San Diego | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock San Francisco | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock San Francisco Financial District | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Santa Barbara | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Santa Monica | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Sausalito | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Silicon Valley | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Solana Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock South San Francisco | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Summerland | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg In Stock Venice Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro 2.5mg San Francisco Financial District | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Mounjaro In Stock (Eli Lilly) | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, NDC: 00002-1506-80 - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Obesity - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Plant Based Medicine - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Precision Preventive Cardiology - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Atherton - Mounjaro 2.5mg In Stock Atherton | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Avila Beach - Mounjaro 2.5mg In Stock Avila Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Balboa Island - Mounjaro 2.5mg In Stock Balboa Island | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Bel Air - Mounjaro 2.5mg In Stock Bel Air | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Beverly Glen - Mounjaro 2.5mg In Stock Beverly Glen | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Beverly Hills - Mounjaro 2.5mg In Stock Beverly Hills | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Brentwood - Mounjaro 2.5mg In Stock Brentwood | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Bridgeport - Mounjaro 2.5mg In Stock Bridgeport | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Calabasas - Mounjaro 2.5mg In Stock Calabasas | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Carpinteria - Mounjaro 2.5mg In Stock Carpinteria | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Chappaqua - Mounjaro 2.5mg In Stock Chappaqua | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Corona Del Mar - Mounjaro 2.5mg In Stock Corona Del Mar | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Crystal Cove - Mounjaro 2.5mg In Stock Crystal Cove | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Cupertino - Mounjaro 2.5mg In Stock Cupertino | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Del Mar Heights - Mounjaro 2.5mg In Stock Del Mar Heights | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Foster City - Mounjaro 2.5mg In Stock Foster City | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Garden City - Mounjaro 2.5mg In Stock Garden City | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Goleta - Mounjaro 2.5mg In Stock Goleta | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Greenwich - Mounjaro 2.5mg In Stock Greenwich | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Hidden Hills - Mounjaro 2.5mg In Stock Hidden Hills | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Hidden Valley - Mounjaro 2.5mg In Stock Hidden Valley | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine La Jolla Shores - Mounjaro 2.5mg In Stock La Jolla Shores | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Laguna Beach - Mounjaro 2.5mg In Stock Laguna Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Larkspur - Mounjaro 2.5mg In Stock Larkspur | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Long Island - Mounjaro 2.5mg In Stock Long Island | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Malibu - Mounjaro 2.5mg In Stock Malibu | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Manhattan - Mounjaro 2.5mg In Stock Manhattan | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Manhattan Financial District - Mounjaro 2.5mg In Stock Manhattan Financial District | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Marin County - Mounjaro 2.5mg In Stock Marin County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Massapequa - Mounjaro 2.5mg In Stock Massapequa | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Mill Valley - Mounjaro 2.5mg In Stock Mill Valley | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Monarch Beach - Mounjaro 2.5mg In Stock Monarch Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Montecito - Mounjaro 2.5mg In Stock Montecito | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Mountain View - Mounjaro 2.5mg In Stock Mountain View | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Nassau County - Mounjaro 2.5mg In Stock Nassau County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Newport Beach - Mounjaro 2.5mg In Stock Newport Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Newport Coast - Mounjaro 2.5mg In Stock Newport Coast | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Pacific Palisades - Mounjaro 2.5mg In Stock Pacific Palisades | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Patchogue - Mounjaro 2.5mg In Stock Patchogue | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Rancho Bernardo - Mounjaro 2.5mg In Stock Rancho Bernardo | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Rancho Palos Verdes - Mounjaro 2.5mg In Stock Rancho Palos Verdes | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Rancho Peñasquitos - Mounjaro 2.5mg In Stock Rancho Penasquitos | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Rancho Santa Fe - Mounjaro 2.5mg In Stock Rancho Santa Fe | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Redwood City - Mounjaro 2.5mg In Stock Redwood City | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine San Mateo County - Mounjaro 2.5mg In Stock San Mateo County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Santa Barbara - Mounjaro 2.5mg In Stock Santa Barbara | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Sausalito - Mounjaro 2.5mg In Stock Sausalito | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Silicon Valley - Mounjaro 2.5mg In Stock Silicon Valley | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine South San Francisco - Mounjaro 2.5mg In Stock South San Francisco | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Suffolk County - Mounjaro 2.5mg In Stock Suffolk County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Summerland - Mounjaro 2.5mg In Stock Summerland | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Venice Beach - Mounjaro 2.5mg In Stock Venice Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Wall Street - Mounjaro 2.5mg In Stock Wall Street | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Prediabetes - Concierge Medicine Westport - Mounjaro 2.5mg In Stock Westport | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Atherton - Mounjaro 2.5mg In Stock Atherton | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Avila Beach - Mounjaro 2.5mg In Stock Avila Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Balboa Island - Mounjaro 2.5mg In Stock Balboa Island | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Bel Air - Mounjaro 2.5mg In Stock Bel Air | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Beverly Glen - Mounjaro 2.5mg In Stock Beverly Glen | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Beverly Hills - Mounjaro 2.5mg In Stock Beverly Hills | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Brentwood - Mounjaro 2.5mg In Stock Brentwood | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Bridgeport - Mounjaro 2.5mg In Stock Bridgeport | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Calabasas - Mounjaro 2.5mg In Stock Calabasas | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Carpinteria - Mounjaro 2.5mg In Stock Carpinteria | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Chappaqua - Mounjaro 2.5mg In Stock Chappaqua | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Corona Del Mar - Mounjaro 2.5mg In Stock Corona Del Mar | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Crystal Cove - Mounjaro 2.5mg In Stock Crystal Cove | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Cupertino - Mounjaro 2.5mg In Stock Cupertino | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Del Mar Heights - Mounjaro 2.5mg In Stock Del Mar Heights | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Foster City - Mounjaro 2.5mg In Stock Foster City | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Garden City - Mounjaro 2.5mg In Stock Garden City | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Goleta - Mounjaro 2.5mg In Stock Goleta | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Greenwich - Mounjaro 2.5mg In Stock Greenwich | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Hidden Hills - Mounjaro 2.5mg In Stock Hidden Hills | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Hidden Valley - Mounjaro 2.5mg In Stock Hidden Valley | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine La Jolla Shores - Mounjaro 2.5mg In Stock La Jolla Shores | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Laguna Beach - Mounjaro 2.5mg In Stock Laguna Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Larkspur - Mounjaro 2.5mg In Stock Larkspur | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Long Island - Mounjaro 2.5mg In Stock Long Island | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Malibu - Mounjaro 2.5mg In Stock Malibu | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Manhattan - Mounjaro 2.5mg In Stock Manhattan | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Manhattan Financial District - Mounjaro 2.5mg In Stock Manhattan Financial District | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Marin County - Mounjaro 2.5mg In Stock Marin County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Massapequa - Mounjaro 2.5mg In Stock Massapequa | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Mill Valley - Mounjaro 2.5mg In Stock Mill Valley | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Monarch Beach - Mounjaro 2.5mg In Stock Monarch Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Montecito - Mounjaro 2.5mg In Stock Montecito | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Mountain View - Mounaro 2.5mg In Stock Mountain View | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Nassau County - Mounjaro 2.5mg In Stock Nassau County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Newport Beach - Mounjaro 2.5mg In Stock Newport Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Newport Coast - Mounjaro 2.5mg In Stock Newport Coast | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Pacific Palisades - Mounjaro 2.5mg In Stock Pacific Palisades | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Patchogue - Mounjaro 2.5mg In Stock Patchogue | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Rancho Bernardo - Mounjaro 2.5mg In Stock Rancho Bernardo | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Rancho Palos Verdes - Mounjaro 2.5mg In Stock Rancho Palos Verdes | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Rancho Peñasquitos - Mounjaro 2.5mg In Stock Rancho Penasquitos | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Rancho Santa Fe - Mounjaro 2.5mg In Stock Rancho Santa Fe | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Redwood City - Mounjaro 2.5mg In Stock Redwood City | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine San Mateo County - Mounjaro 2.5mg In Stock San Mateo County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Santa Barbara - Mounjaro 2.5mg In Stock Santa Barbara | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Sausalito - Mounjaro 2.5mg In Stock Sausalito | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Silicon Valley - Mounjaro 2.5mg In Stock Silicon Valley | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine South San Francisco - Mounjaro 2.5mg In Stock South San Francisco | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Suffolk County - Mounjaro 2.5mg In Stock Suffolk County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Summerland - Mounjaro 2.5mg In Stock Summerland | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Venice Beach - Mounjaro 2.5mg In Stock Venice Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Concierge Medicine Westport - Mounjaro 2.5mg In Stock Westport | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Preventive Cardiology- Concierge Medicine Wall Street - Mounjaro 2.5mg In Stock Wall Street | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Atherton - Mounjaro 2.5mg In Stock Atherton | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Avila Beach - Mounjaro 2.5mg In Stock Avila Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Balboa Island - Mounjaro 2.5mg In Stock Balboa Island | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Bel Air - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Beverly Glen - Mounjaro 2.5mg In Stock Beverly Glen | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Beverly Hills - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Brentwood - Mounjaro 2.5mg In Stock Brentwood | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Bridgeport - Mounjaro 2.5mg In Stock Bridgeport | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Calabasas - Mounjaro 2.5mg In Stock Calabasas | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Carpinteria - Mounjaro 2.5mg In Stock Carpinteria | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Chappaqua - Mounjaro 2.5mg In Stock Chappaqua | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Corona Del Mar - Mounjaro 2.5mg In Stock Corona Del Mar | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Crystal Cove - Mounjaro 2.5mg In Stock Crystal Cove | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Cupertino - Mounjaro 2.5mg In Stock Cupertino | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Del Mar Heights - Mounjaro 2.5mg In Stock Del Mar Heights | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Foster City - Mounjaro 2.5mg In Stock Foster City | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Garden City - Mounjaro 2.5mg In Stock Garden City | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Greenwich - Mounjaro 2.5mg In Stock Greenwich | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Hidden Hills - Mounjaro 2.5mg In Stock Hidden Hills | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Hidden Valley - Mounjaro 2.5mg In Stock Hidden Valley | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine La Jolla Shores - Mounjaro 2.5mg In Stock La Jolla Shores | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Laguna Beach - Mounjaro 2.5mg In Stock Laguna Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Larkspur - Mounjaro 2.5mg In Stock Larkspur | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Long Island - Mounjaro 2.5mg In Stock Long Island | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Malibu - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Malibu - Mounjaro 2.5mg In Stock Malibu | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Manhattan - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Manhattan Financial District - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Marin County - Mounjaro 2.5mg In Stock Marin County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Massapequa - Mounjaro 2.5mg In Stock Massapequa | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Mill Valley - Mounjaro 2.5mg In Stock Mill Valley | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Monarch Beach - Mounjaro 2.5mg In Stock Monarch Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Montecito - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Montecito - Mounjaro 2.5mg In Stock Montecito | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Mountain View - Mounajro 2.5mg In Stock Mountain View | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Nassau County - Mounjaro 2.5mg In Stock Nassau County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Newport Beach - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Newport Coast - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Pacific Palisades - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Patchogue - Mounjaro 2.5mg In Stock Patchogue | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Rancho Bernardo - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Rancho Palos Verdes - Mounjaro 2.5mg In Stock Rancho Palos Verdes | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Rancho Peñasquitos - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Rancho Santa Fe - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Redwood City - Mounjaro 2.5mg In Stock Redwood City | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine San Mateo County - Mounjaro 2.5mg In Stock San Mateo County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Santa Barbara - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Sausalito - Mounjaro 2.5mg In Stock Sausalito | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Silicon Valley - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine South San Francisco - Mounjaro 2.5mg In Stock South San Francisco | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Suffolk County - Mounjaro 2.5mg In Stock Suffolk County | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Venice Beach - Mounjaro 2.5mg In Stock Venice Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Wall Street - Mounjaro 2.5mg In Stock Wall Street | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Concierge Medicine Westport - Mounjaro 2.5mg In Stock Westport | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Mounjaro 2.5mg In Stock | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Mounjaro 2.5mg In Stock Beverly Hills | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Mounjaro 2.5mg In Stock Newport Beach | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711, Weight Loss - Mounjaro 2.5mg In Stock Newport Coast | ApoThera - Concierge Specialty Pharmacy | Phone: (949)387-7711